Background: African American individuals (AAs) are 2 to 3 times more likely to have MGUS compared to European American individuals. There is a gap in our understanding as to why AAs are more predisposed to develop MGUS and what biological pathways drive the disease from its precursor state to overt MM. Studies on biology and biomarker discovery involving AAs in MGUS are limited. Protein expression patterns, as opposed to alterations in DNA or RNA expression, can identify post-translational modifications and determine protein-protein interactions, all of which contribute to the complexity and heterogeneity that underlines MM biology. In this study, we used mass-spectrometry-based serum proteomics profiling of AAs and Whites with MGUS and MM to identify new biomarker signatures that predict disease progression and help advance our understanding of the biological mechanisms underlying the racial disparity that exists in this disease.
Methods: In this feasibility study, comprehensive profiling of serum proteins by DIA mass spectrometry proteomics (with quantification of over 500 proteins) was completed on 20 MGUS and 20 MM serum samples belonging to untreated patients (equal number of AAs and Whites in each disease state; matched for age and sex). For the logistic regression, missing values were set to 0 and all other values were set to 1 while testing for association between the increased presence or absence of a protein and MGUS/ MM status accounting for sex, age, batch, and race were covariates. Those proteins meeting an FDR < 0.05 were retained for pathway enrichment and network analysis (REACTOME, KEGG). sPLS-DA was used to identify the set of proteins that optimizes discrimination between MGUS and MM .
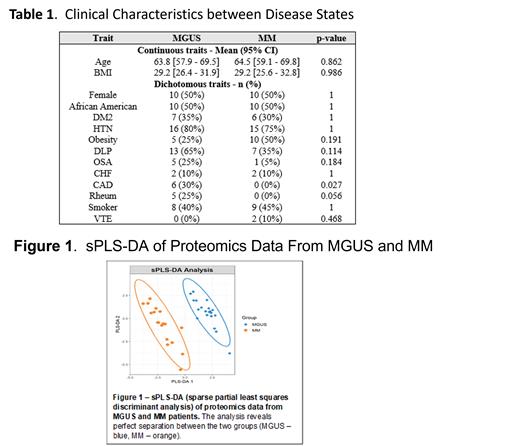
Results: There was a higher proportion of patients with coronary artery disease and rheumatologic conditions in the MGUS cohort (See Table 1). Although there was no difference in BMI by disease state, AAs did have a significantly higher BMI compared to Whites (33.1 vs. 26.7; p=0.008). The exploratory analysis identified 98 proteins that significantly differed between the two disease states (Figure 1), thus confirming the existence of distinct proteomic profiles and biological differences between MGUS and MM. No significant difference was observed between sex and racial groups. The significant proteins were used in downstream analysis to identify biological networks and pathways that are most informative. Pathways that were important differentiators between MGUS and MM were innate inflammation, immune regulation, homeostasis, platelet activation and insulin growth factor regulation The most strongly enriched pathways were glycolysis and gluconeogenesis (p= 1.5 x10 -7) and cholesterol metabolism (p=8.05x10 -6). These biological networks appear to play roles in thrombo-inflammation which is known to have profound implications in cancer and may be a factor contributing to progression from MGUS to MM. Interestingly, there were 3 patients with MGUS who clustered closer to the MM samples. Clinically, these patients had significantly higher involved light chains (178.21 vs. 28.65 mg/L; p=0.0193) and M protein values (0.9 vs. 0.4 g/dL; p=0.00744) than the rest of the patients with MGUS.
Conclusions: This work suggests that there are clear quantitative proteomic alterations that occur early during myelomagenesis. While certainly not conclusive, these results reveal novel cellular and molecular mechanisms that may contribute to disease progression. No race-based differences in proteomic profiles and molecular pathways were detected at this stage, maybe due to the small sample size. We plan to expand our preliminary work by increasing the number of MGUS and MM samples and by including a subset of samples from patients with smoldering MM to develop a proteomic risk model that can predict risk of progression by identifying patients with smoldering MM who harbor more “MGUS-like” profiles versus patients with more “MM-like” profiles.
Disclosures
Varga:ARCLLEX: Research Funding; MMRF: Honoraria. Voorhees:Sanofi: Membership on an entity's Board of Directors or advisory committees; Nervianos Medical Sciences: Research Funding; Regeneron: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Novartis: Consultancy; GSK: Consultancy, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Bhutani:Amgen: Research Funding; Bristol-Myers Squibb/Celgene: Research Funding; Janssen Research & Development: Research Funding; Adaptive Biotechnologies: Research Funding; Takeda: Research Funding.